inhaled budesonide for covid 19
promising results from phase 2 trial using a common asthma medication
cliff notes: budesonide (pulmicort turbohaler) showed highly promising and strongly statistically significant reduction in progression of mild covid to severe presentation requiring an urgent care visit. it reduced symptoms and sped recovery. drop in incidence of urgent care visits was on the order of 80-90% vs control group.
this was a drug trial in the UK using inhaled budesonide, a widely available cortico-steroid used to treat (among other things) asthma. it’s a safe, easy to administer drug that can be purchased for $55 at costco or publix making it an attractive option for use. the study was conceived because it was noticed that people with reparatory diseases, who would have been expected to have higher and more severe incidence of SARS-COV19, in fact showed the opposite. thus it was hypothesized that drugs used to treat those conditions might be effective against covid-19.
this STUDY was published in “the lancet” and the trial is registered with the FDA at clintrials.gov HERE.
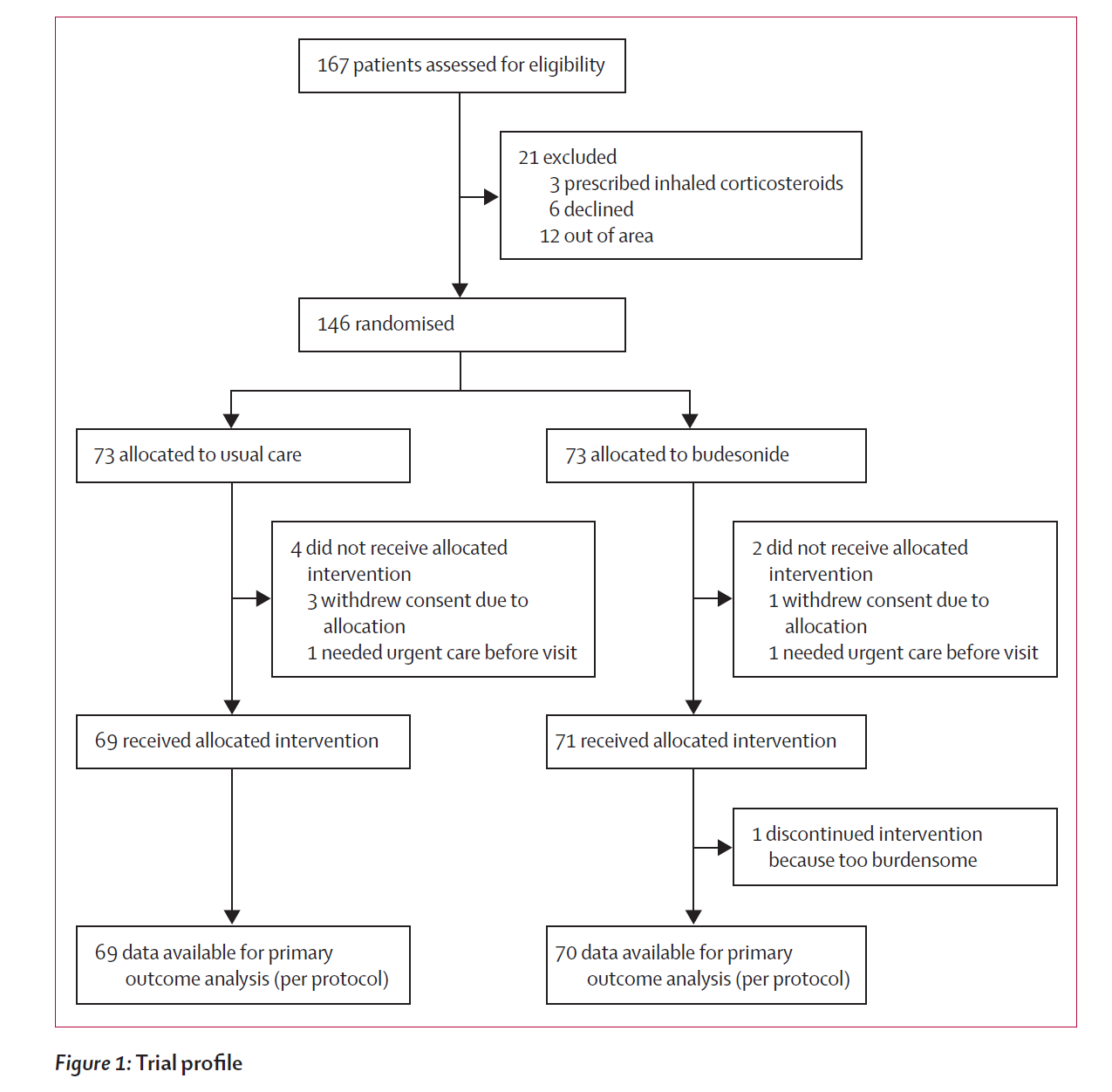
study design was straightforward with patients with early onset covid randomized into age and comorbidity stratified cohorts and then one treated with standard of care and the other with twice a day inhalations of budesonide via turbohaler, a small, portable device most people will find familiar. dosage was 800μg per treatment (BID).
enrollment worked like this:
the post randomization profiles look good and highly comparable with no meaningful divergences. (see p 4 of the study). primary outcome for the trial was set as “covid-19 related urgent care visits” and a number of secondary symptomatic markers. the results looked extremely promising and the method of action (reduction in lung inflammation) seems both plausible and a direct intervention with low risk.
results:
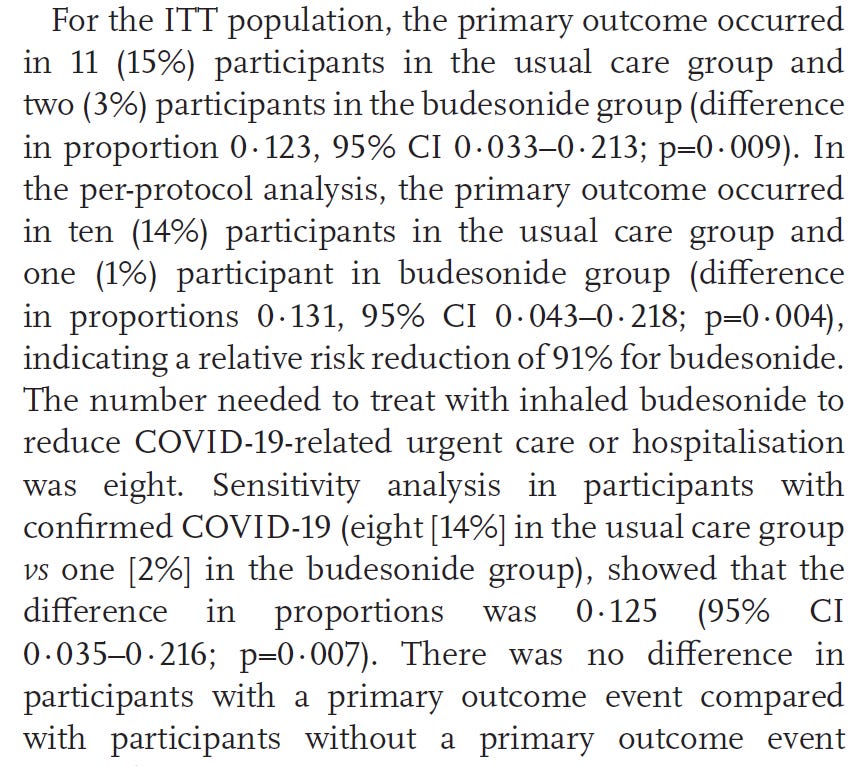
keep in mind in reading this that primary outcome was a trip to the ER/urgent care, so lower incidence of primary outcome is better.
ITT = intent to treat this is the patient population that underwent randomization. per protocol (n=139) are the patients that completed the protocol without withdrawing or being excluded for for actually receiving the allocated intervention.
per protocol is likely the better measure of overall outcomes here but both are included for completeness.
ITT: 15% of the control group sought urgent care. 3% of active arm did. 80% reduction in trips to UC/ER. p=0.009
PP: 14% of control group sought urgent care. 1% of active arm did. 93% reduction in trips to UC/ER. p= 0.004
these are major reductions and extremely good p values (anything below 0.05 is considered statistically significant, below 0.01 is really excellent).
participants in the active arm saw more rapid and more complete recoveries. divergence by day 14 was large with only 7 treatment arm patients still “at risk” vs 21 in control. (67% reduction) this rose to 86% reduction by day 18 and 90% by day 28.
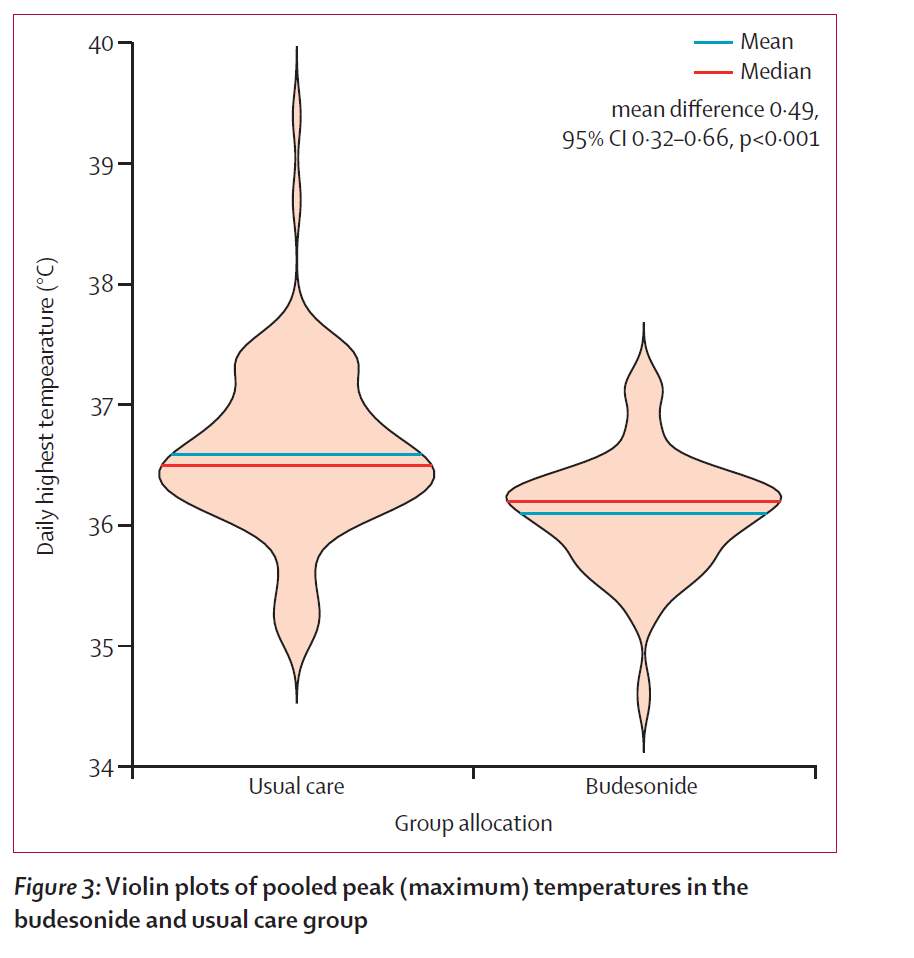
they also saw lower and less extreme daily maximum temperatures. p value on this divergence was <0.001 which is as good a number as one is ever going to see in a trial this size.
conclusions:
all in all, this was a VERY promising trial. results were strong despite a small n and robust across multiple markers both subjective and objective. this is a similar treatment to the one my doctor has been prescribing (via nebulizer) to patients with good results. i’m not here to give you definitive medical advice, but i really would recommend showing this study to your doctor and asking them about it should you contract covid (or perhaps before as doctors miss data all the time and this may be of real help to people). make the choice that’s right for you, but this is a choice that i think ought to be on your list to consider.
the ability to drop urgent care trips by 80-90% with a simple, safe intervention that is easy to administer and cheap to provide is a big deal. presuming these results validate and remain robust, it’s a back breaker for covid threat.
my one quibble with the study is that the control arm was not given a sham inhaler and thus one could argue that there was a placebo effect from using one that remained uncontrolled for. i suspect the risk that this tainted the study in a problematic manner is low, but it pays to be complete and a future study really ought to include a placebo nebulizer.
but all in all, this looks promising and robust. they look to be onto a winner here.






Thank you for coming back to the discussion!
It is a crime that western governments have suppressed the study and use of treatments in favor of vaccines with known severe short-term side effects and unknown long-term side effects. From @Covid19Crusher “The surprising map of the number of completed Covid-19 clinical trials by country to date. #1: Iran (109) #2: USA (43) #3: Spain (22) #4: Brazil/Russia (15) Shame on the West.” https://covid-nma.com/dataviz/
My child is a student at Notre Dame. It currently has a vaccination site on campus. The day before vaccinating began ND sent an email to the students coercing them to take the vaccine with a promise to relax some of the draconian restrictions and stating vaccination is required to return to campus in the fall.
My child reported to me today that half of the students she knows who have had the first shot have had to miss a day of activities stating they felt worse than they did in quarantine with the virus. The best part: students have been told not to report these symptoms on the ND symptom tracker because they would be considered virus infection symptoms and go into quarantine - no side effects “Here!.”
ND taught me how to question, research, read with intent, analyze, and act on moral and ethical principles. Therefore, I ask myself
If a vaccine helps the recipient to not get ill or get ill with less severe symptoms, can an interaction with an unvaccinated person affect the health of that person?
If someone already had an illness, is there is a historical or scientific need for that person to be vaccinated as the vaccines have no more proof of long-term effectiveness as natural immunity?
If a vaccine has less than one year of safety data since first injected into living animals or people, often causes miserable side effects that are worse than the virus itself for a particular group of people who have already had high exposure to it, why should that group be mandated to take it?
My child has asked these questions (and, thankfully, knows the correct answers), but my child did not learn to do that at ND. Two questions I am asking myself are what has happened to my alma mater and should I continue to pay my child’s tuition?
It is a crime that western governments have suppressed studies of and are denying treatments in favor of vaccines with severe short-term side effects and unknown long-term side effects. From @CovidCrsher (https://covid-nma.com/dataviz/) "The surprising map of the number of completed Covid-19 clinical trials by country to date. #1: Iran (109) #2: USA (43) #3: Spain (22) #4: Brazil/Russia (15) Shame on the West."
I am trying to get the following post vaccination experiences at a college campus exposed:
My child is a student at Notre Dame. It currently has a vaccination site on campus. The day before vaccinating began ND sent an email to the students coercing them to take the vaccine with a promise to relax some of the draconian restrictions and stating vaccination is required to return to campus in the fall.
My child reported to me today that half of the students she knows who have had the first shot have had to miss a day of activities stating they felt worse than they did in quarantine with the virus. The best part: students have been told not to report these symptoms on the ND symptom tracker because they would be considered virus infection symptoms and go into quarantine - no side effects “Here!.”
ND taught me how to question, research, read with intent, analyze, and act on moral and ethical principles. Therefore, I ask myself
If a vaccine helps the recipient to not get ill or get ill with less severe symptoms, can an interaction with an unvaccinated person affect the health of that person?
If someone already had an illness, is there is a historical or scientific need for that person to be vaccinated as the vaccines have no more proof of long-term effectiveness as natural immunity?
If a vaccine has less than one year of safety data since first injected into living animals or people, often causes miserable side effects that are worse than the virus itself for a particular group of people who have already had high exposure to it, why should that group be mandated to take it?
My child has asked these questions (and, thankfully, knows the correct answers), but my child did not learn to do that at ND. Two questions I am asking myself are what has happened to my alma mater and should I continue to pay my child’s tuition?