are we pfinding pfizer pfraud? (part 1)
the document trail from the pfizer data dump has a number of worrying signs
the pfizer data dumps are massive and disordered. they drop 50k pages without regard to completeness or organization.
here, have a huge box of who knows what!
this is a longstanding legal trick. and it works well. it’s the time honored fashion in which big companies bury small legal teams and create a $3 million bill just to sort and read the stuff.
but here’s a fun little wrinkle: this time is not going to be like that.
battalions of interested parties are all over it. the documents are posted and reading and sorting them is crowdsourced. and many hands make for light work. and a lot of very skilled sleuths are at work here.
no law firm in history has ever had an expert team like this. never.
the FDA and pfizer fought tooth and claw NOT to release this.
i have ZERO doubt that they are dumping all the least awful and incriminating things first. keep in mind these folks wanted to take 75 years to disclose this. only a court order pried it loose. but even with this first set of boxes, it’s starting to become apparent why they did not want anyone poking around.
they knew all sorts of things they failed to disclose and many are doing good work on this.
but what if the trial itself was pfraud?
because THAT would be seismic. it would not only severely implicate the FDA and pfizer alike, it would likely invalidate the liability protection granted under EUA and suddenly bourla and his merry band of vaxx aficionados might find themselves accountable. bigly.
and wouldn’t that be interesting?
so if this looks like a plausible possibility, we really ought to dig in like busy little mice and see what all we can winkle out. fortunately, some busy little mice have already been doing so. and what they found is, well, pfrightening.
obviously, it’s early, we’re just getting going, we have not heard the other side of this story yet nor afforded them the opportunity to offer explanation and/or defense, but as someone who, like jikkyleaks, has been around lots of study enrollment, at first pass this pattern stinks like a red lobster dumpster. in houston. in august.
so let’s look:
pfizer recruited ~44k people into their vaccine study in record time. it was, as far as i know, the fastest clinical trial enrollment of this magnitude in human history. by a lot. they had 270 clinical sites numbered 1001 to 1270.
and several look to have some problems with their alleged patient process.
the biggest site was 1231 in argentina.

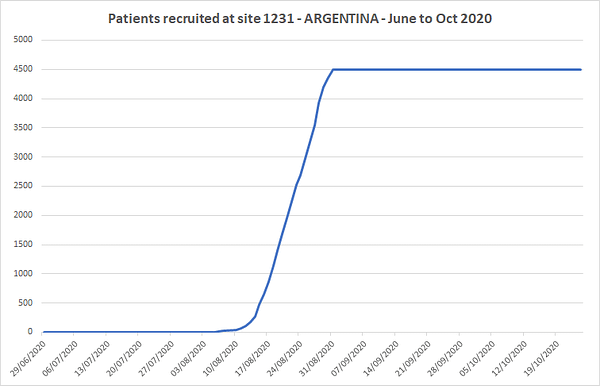
4,501 patients in 3 weeks at military central hospital.
that’s a helluva thing.
now, anyone who has ever run a clinical trial is already probably jumping up and down like this:
for those who have not, allow me to explain: this is basically impossible. i don’t care who you are and what your team is.
if this really happened, it would be a wonder of the world and they should publish the process with pride and win 27 different prizes for it. stuff should be named after them.
but i doubt this will end up being the case.
they claim to have enrolled 7 days a week for 3 weeks with zero gaps. each patient requires a 250 page case report form. the lead investigator seems to have been fernando polack.
if, indeed, the best way to get things done is to give them to busy people, then this was a great choice, because from the look of things, fernando is one busy fellah.
and connected up the wazoo to boot.
he also works with vanderbilt, the FDA, and the infant foundation, funded by the gates foundation and the NIH.
and somehow, during this same period, he ran a study on convalescent plasma.
all of this is odd as polack is a pediatrician by training and seems an odd choice to head so much work in adults.
alas, a subsequent systematic review and meta-analysis failed to confirm these findings, noting “very serious imprecision concerns”.
oopsie.
we learn more about polack: (boldface mine)
Fernando has been commercially involved in clinical trials since 2014 when he was the director of Unitrials SA in 2014. This changed its name to Infant Trials SA in 2015.
iTRIALS SA was formed the following year. His co-directors are biochemist Silvina Andrea Coviello and Fernando’s guitarist son Leandro.
There are now four iTRIALS subsidiary LLC companies registered in Florida, c/o the offices of Cervetta-Lapham & Associates.
iTRIALS is a site management organisation (SMO).
“iTRIALS assists sponsors, CROs [Contract Research Organisations] and investigators in all aspects of clinical trials to help ensure that goals and expectations are timely and successfully met with international quality standards.”
“iTRIALS identifies high volume clinical research sites of extraordinary recruitment potential and high-quality staff.”
iTRIALS shares its offices with another of Fernando’s babies – Fundación INFANT
Meanwhile, on 10 July 2020, without any prior announcement, Fernando Polack and Nicolás Vaquer, CEO of Pfizer in Argentina, called on President Fernandez to tell him that Argentina had been selected by Pfizer to conduct Phase III clinical trials of their Covid Vaccine. Polack had taken part in Zoom calls with Pfizer since May that year.
Infobae reported Fernando proudly saying
“…if you have the scientific privilege of participating in the evaluation of a vaccine, you contribute to potentially better position your country in the waiting line for distribution.”
This placebo-controlled trial was arranged with military precision. Fleets of taxis were booked to ferry volunteers between their homes and the vaccine clinic at Hospital Militar Central in the northeast of the city. Volunteers were each given a tablet with a pre-installed app to record pre-defined symptoms.
About 5,800 volunteers were enrolled, half getting the active vaccine. This is almost 4 times more than the next largest centre in this trial. Amazingly 467 doctors were almost instantly signed up and trained as assistant investigators in the study. Fernando was in command as Pfizer’s Principal Investigator.
The first article about the Pfizer-sponsored multinational ground-breaking study was published in the New England Journal of Medicine on 31 December 2020, with Fernando as first author. Is this the first paper in NEJM with the owner of an SMO as first author?
recruiting, coordinating, and training 467 doctors this quickly is DEEPLY implausible as is having gotten all the patient flow through them including eligibility, inclusion criteria, etc. this would be a marvel at any medical center on earth. in BA, it’s a hat trick of concatenated miracles.
he got the word on 10 july. he was enrolling by the beginning of august, less than 3 weeks later and by 10 august was into the steep part of the ramp. all 4501 were done by end august less than 6 weeks after getting the go to start.
sorry, but i am finding this WAY past anywhere my credulity will stretch.
(note that the 5800 number varies from the 4501 at site 1231, and i think i may know why. (more on this later))
there are some additional suspicious inklings here: (raised back in march by dr david healy)
The second author was Dr Stephen Thomas of the State University of New York, Upstate Medical University, Syracuse. In a BMJ podcast, Dr Thomas bills himself as the Lead Principal Investigator. His academic centre enrolled 364 volunteers.
Despite leading this project, Dr Thomas makes it very clear that he was only allowed access to analyses of aggregated data and didn’t know when or if Pfizer would release the raw data. It is not clear how much or how Pfizer paid for SUNY participation in the trial.
Our question is: Who ran the Buenos Aires vaccine trial, and where did the money go?
indeed, these are excellent questions. this site alone comprises over 10% of enrollment (probably more like 13%, more soon) and the whole story around it looks outlandishly implausible. in a locale famous for fraud and non-extradition.
huh.
this might warrant a bit more investigation, no?
especially given the uncertainty on just who ran this and what their conflicts of interest were.
healy speculates:
it’s not at all clear how the money flowed here and having the owner of the SMO running the trial also be the chief investigator in that trial is an astonishing conflict of interest and lacks the rudimentary checks and balances one would normally seek.
it might, for example, lead to some naughty games being played to get the results you want. because, who would know?
and ventavia has had some problems with it’s site for the pfizer trial:
The staff mostly women, dressed in fetching slate gray uniforms, were hired from local fast-food outlets and other settings. Most had no training. Those giving the vaccines should have had a healthcare background but didn’t and some had no training prior to starting – taking courses afterwards to ensure boxes were ticked.
um, yikes?
and whistleblowers had a lot to say.
and yet they were unaudited.
this finds worrysome confluence with what has always looked to me like a pile of classification games played in the trial itself.
astonishing numbers of “adverse events” were declared “not vaccine related” and the AE’s in the placebo arm looked WAY out of line for saline injections.
and far more people were dropped from the active arm than the placebo. enough that you could have completely hidden a high side effect profile if this was done selectively.
discussed in more detail here:
altogether, this generates a serious pattern that smacks of fraud and manipulation.
it’s certainly enough to demand a full forensic investigation. the fact the the FDA did not audit the BA center that did 10-13% of the entire trial enrollment despite these huge red flags is outlandish.
there’s no way they made a mistake like that.
it was either dereliction so blatant as to seem implausible or a decision not to go looking for fear of what might be found.
this alone should put this whole approval on pause for review.
but the plot thickens.
enter “site 4444.”
(see next companion piece. these were too long to send as a single email)











And what about the PRECLINICAL studies? I have not heard one word about any that were done anywhere. I worked 37 years as a data technician for MPI Research, and this going straight to human (clinical) testing is simply not done! Before one ever tests anything in humans there is a whole battery of preclinical animal studies that need to be conducted, of varying lengths and in different species, looking at things like carcinogenicity, neurotoxicity, reproductive problems, cardiovascular problems. Where is the data for these studies? Who did them? (Hint: It wasn't MPI!)
That these trials were fraudulent should be the expected outcome given that there has never been evidence of efficacy.
https://roundingtheearth.substack.com/p/vrbpac-presentation-the-ultimate?s=w