many likely remember the changes to decongestants mandated back in 2006 when longstanding drug pseudoephedrine was replaced by phenylephrine because “trailer chemists were using decongestants to make meth.”
at the time, folks largely seemed OK with this and assumed that well, yeah, sure, maybe the new one is not as good, but it makes sense.
but did it? did it reduce meth one whisker? not that i am aware.
and did this new ingredient even actually work at all/was it even evaluated by any remotely modern standard of testing as a decongestant before it was sold for 17 years in 100’s of millions of boxes of over the counter medicine?
well, apparently not.
this whole thing, like so many aspects of the FDA and the war on drugs was just a sham rooted in murky fact claims, supposition, and utopian reality denial.
apparently, there was no real testing and phenylephrine never worked at all. these products have been fraudulently marketed since they day they hit the shelves and took 80% market share.
as things stand today:
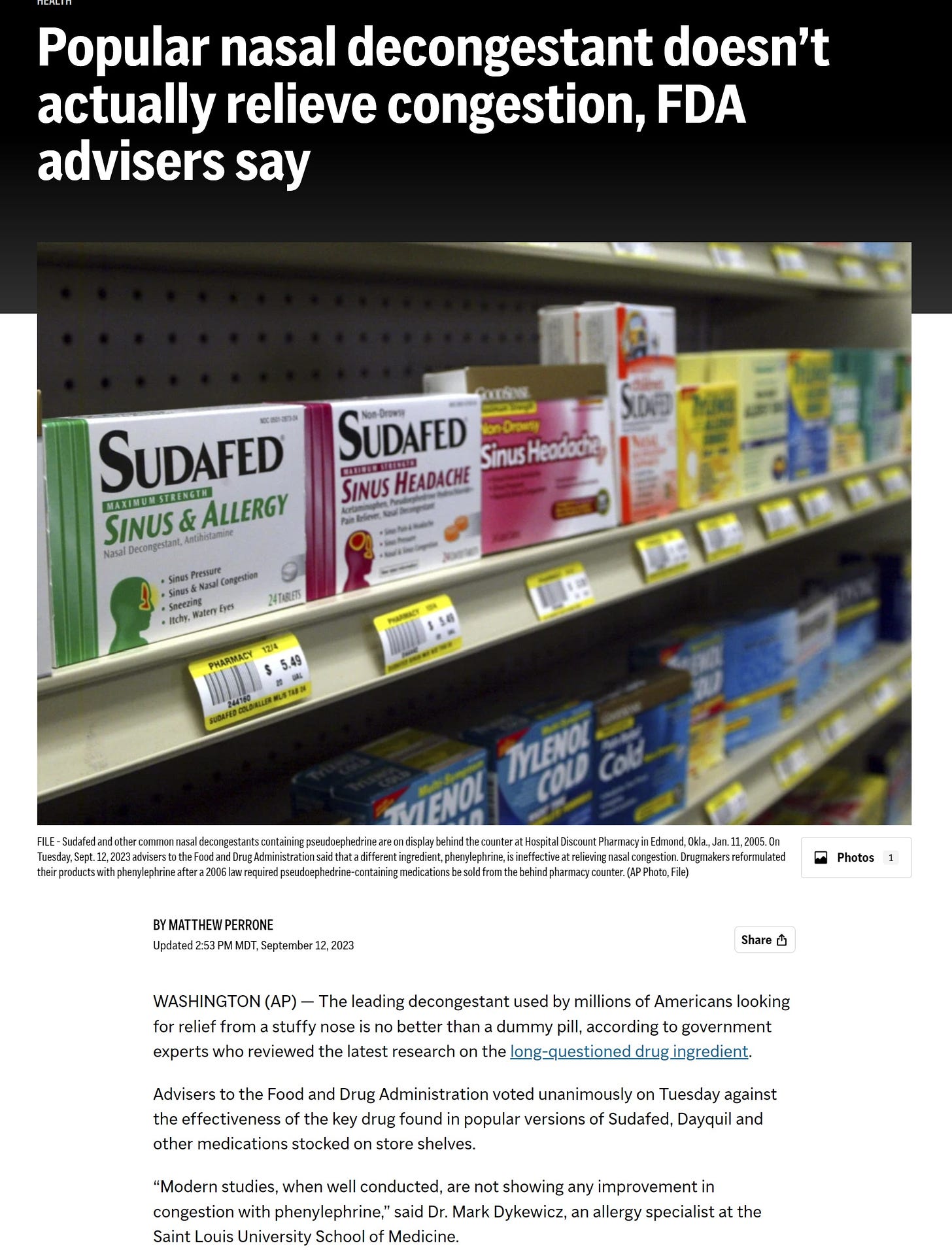
Those original versions of Sudafed and other medicines remain available without a prescription, but they’re less popular and account for about one-fifth of the $2.2 billion market for oral decongestants. Phenylephrine versions — sometimes labeled “PE” on packaging — make up the rest.
If the FDA follows through on the panel’s recommendations, Johnson & Johnson, Bayer and other drugmakers could be required to pull their oral medications containing phenylephrine from store shelves. That would likely force consumers to switch to the behind-the-counter pseudoephedrine products or to phenylephrine-based nasal sprays and drops.
In that scenario, the FDA would have to work with drugstores, pharmacists and other health providers to educate consumers about the remaining options for treating congestion, panelists said Tuesday.
and if the FDA panel is suddenly pushing it “unanimously” and the AP marketing wire is reporting it, you should be looking for how the fix is going in. because this fact pattern looks suspicious.
because this has been an issue since day 1 and the “studies” used to allow the substitution were always junk.
when looking at this old “grandfathered” product category, there is a lot of dated nonsense that was never really assessed in any meaningful way.
this would perhaps be actual truth in advertising:
but of course, these OTC products needed to survive. too much money was at stake. so they did. but suddenly, this changes and “the science gets unsettled.”
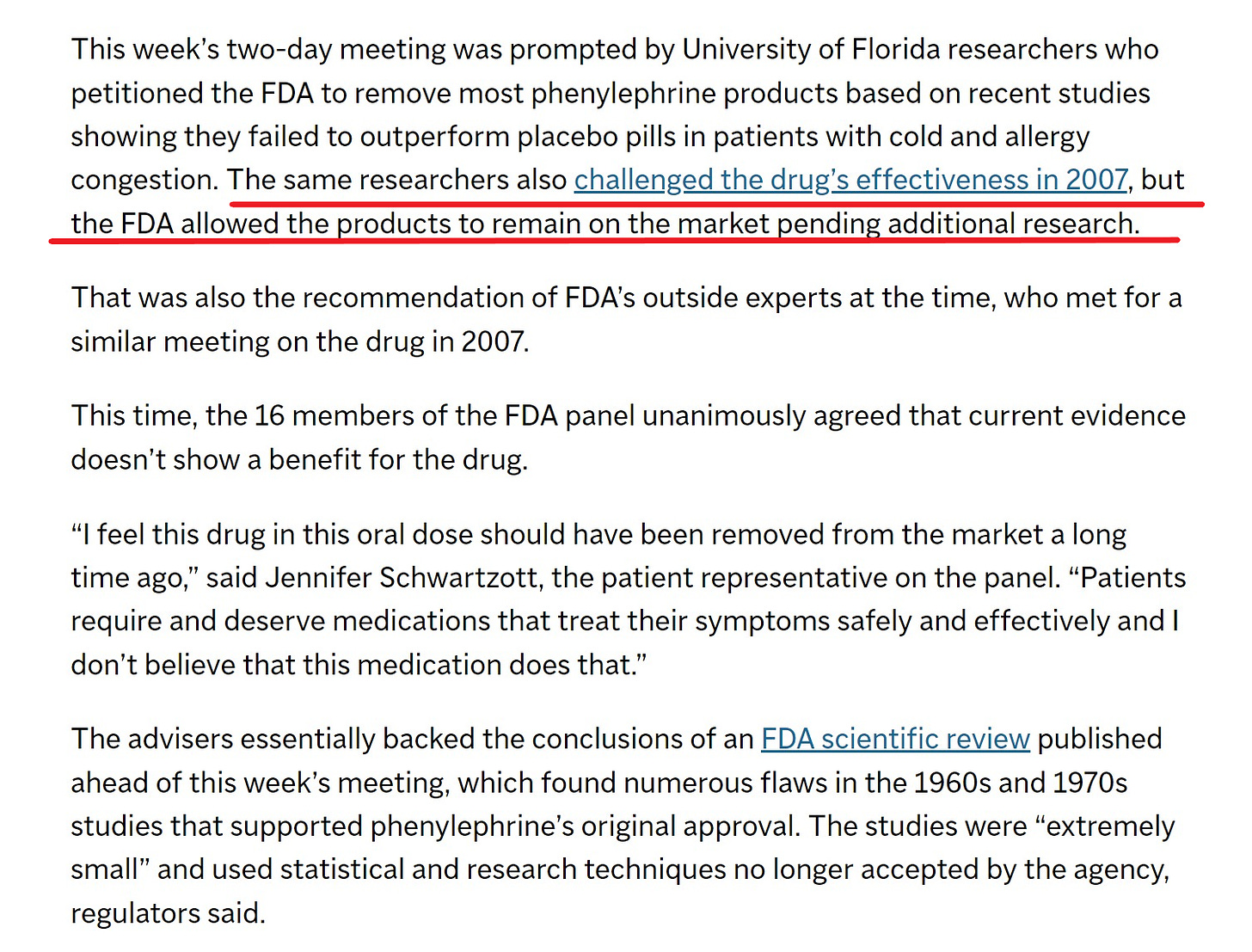
“The bottom line is that none of the original studies stand up to modern standards of study design or conduct,” said Dr. Peter Starke, the agency’s lead medical reviewer.
Additionally, three larger, rigorously conducted studies published since 2016 showed no difference between phenylephrine medications and placebos for relieving congestion. Those studies were conducted by Merck and Johnson & Johnson and enrolled hundreds of patients.
and as is so often the case the interesting question around these abrupt changes in course wind up being “so why now?”
Like many other over-the-counter ingredients, phenylephrine was essentially grandfathered into approval during a sweeping FDA review begun in 1972. It has been sold in various forms for more than 75 years, predating the agency’s own regulations on drug effectiveness.
“Any time a product has been on the market that long, it’s human nature to make assumptions about what we think we know about the product,” said Dr. Theresa Michele, who leads the FDA’s office of nonprescription drugs.
But FDA reviewers said their latest assessment reflects new testing insights into how quickly phenylephrine is metabolized when taken by mouth, leaving only trace levels that reach nasal passages to relieve congestion. The drug appears more effective when applied directly to the nose, in sprays or drops, and those products are not under review.
There’s unlikely to be any immediate impact from Tuesday’s panel vote, which is not binding.
The group’s negative opinion opens the door for the FDA to pull phenylephrine from a federal list of decongestants deemed effective for over-the-counter pills and liquids. The FDA said removing the products would eliminate “unnecessary costs and delay in care of taking a drug that has no benefit.”
and so, here is my wager:
regardless of whether this is “oh, we just assumed that this weaksauce substitution was OK cuz antique studies” vs “we always new this was junk but wanted to prop up a $2 billion product category” we’re now into “hey, we’ve decided to notice and maybe take action.”
wanna bet there is a new something, something oh, say, on patent that someone wants to replace it with?
something that could be incredibly profitable for someone as it instantly replaces phenylephrine across the nation?
by mandate?
yeah.
i’m curious: anyone on team toxoplasmosis have any thoughts on what the candidate might be?
i have no special knowledge here nor even a suspected compound for such a swapperoo, but it sure seems plausible.
or maybe they just want the old ones back because they were more profitable or more popular and they think it grows the market?
one way or the other, let’s face it, the iron law here is non-negotiable:
if you want to chase your tail, follow “the science™.”
if you want know what’s really going on:







Me on my soapbox again, but how can they ban the original Sudafed for being too meth-y… while at the same time overseeing the multibillion dollar adhd drug industry which is all about getting 5 year olds hooked on amphetamines?
The other possibility is, Phenylephrine is useful in treating the next bio weapon they have in the hopper. 😳 With FDA anything is possible.