
there is no medicine but "the medicine™"
regulators have been consumed by covid monomania and every other area of medical endeavor is suffering as a result
this missive from gatopal™ and frequent correspondent jay bhattacharya is spot on. i think people are severely underestimating the magnitude of this effect.

the FDA (and other health regulators) have gone on weird, fundamentalist covidian pilgrimages at the expense of literally everything else.
facilities remain uninspected. trials are not getting approved. ongoing and planned clinical trails are failing or being postponed because the endless circus of randomly rotating regulations make it impossible to enroll and follow up patients and increasingly because the FDA refuses to provide the comments and engagement on study and submission design integral to a successful process.
outside of the vast covidian sphere in which every standard has slipped and every corner has been cut to bring you iffy and substandard products at “warp speed” the rest of medical research is being allowed to die on the vine.
even collaboration and review has turned toxic. to protect its own “metrics” the FDA is handing out complete response letters (CRL) like halloween candy.
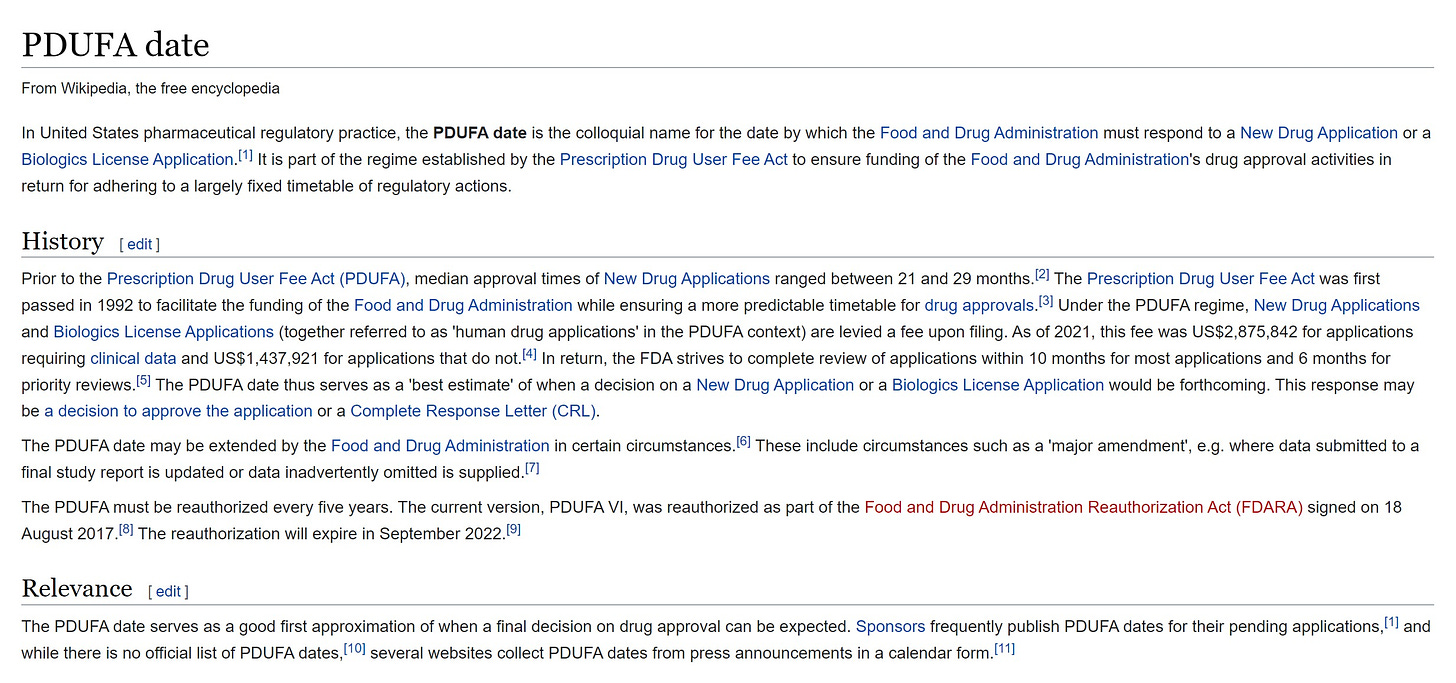
for those not familiar, when you submit a drug or device application to the FDA, it starts a timeclock. this is called a PDUFA date.
failing to respond in a timely fashion gets the FDA dinged so they seek to do so.
this used to run quite well. frankly, under gottlieb, the FDA was truly a gold standard organization and they hit their dates well while providing high quality review and striking a good balance of working with companies on their projects while demanding good study design and requiring sound endpoints and data.
since scott left mid term in april of 2019 to “spend more time with his family” the FDA has been in a downspiral. his departure itself was deeply odd. you basically never see a mid-term resignation, especially from such a popular and effective leader. 2 months earlier, he had tweeted:
“I want to be very clear – I’m not leaving,” he tweeted two months ago. “We’ve got a lot important policy we’ll advance this year."
then he abruptly left without warning or much of an explanation.
since then, there has been no official FDA head and there still is none today.
his departure gave us a warning shot on FDA policy to protect its metrics: they stopped approving EVERYTHING.
just about every PDUFA date that came up right after scott left got a CRL for some minor, picayune issue. it went ripping through the pharma industry like a tornado. it was so unprecedented, so out of proportion, and so prevalent that even wall st pharma analysts were writing pieces about it.
this was the FDA “electing to punt” while they waited to see what the new boss was going to be like. to avoid being penalized for missing dates or being out of step with new leadership, they just bounced everything back and stopped approvals and clearances dead in their tracks to protect the fiefdom, providing yet another fine object lesson in the fashion in which bureaucracies always evolve to be, first and foremost, their own primary constituencies.
(i suspect jay, a professor of economics as well as epidemiology, would agree with me when i say that probably no one should be allowed to take econ 101 without first having studied public choice theory as a prerequisite. it’s like giving someone a handgun without teaching them safety.)
this issue resolved itself as the agency settled into its new ad hoc existence as a regulator without a real boss. “acting heads” are notoriously political and susceptible to suasion from politicians because they are, in effect, still interviewing for their job. they are not confirmed, not tenured, and can be replaced without notice or process.
given the history since then, one wonders if this was seen as a feature and not a bug.
the corners cut, short trial periods, iffy endpoints, eliminated control groups, failure to consider all cause cohort effects, fade, elimination of control groups, and bayesian data tampering by misallocating risk buckets through definitional sleight of hand have been well documented and i won’t belabor them here. clearly, the realm of covid is a world unto itself with standards unlike those of any other.
but the other realms have been suffering mightily from this monomania. everything else has stopped dead.
try to get a new trial started. see what happens. try to get a facility inspected. once more, the CRL’s are flying like some cut rate hip-hop artist “making it rain.” even folks that had priority review and breakthrough status are getting bounced for a bewildering array of reasons. folks that were told one trial was sufficient are being CRL’d and told they need to do a confirmatory.
it’s all gone “darth vader in the cloud city.”
this is the text of a letter the FDA has been sending around to companies asking for guidance on their drug and device studies. these back and forth conversations are how you home in on the best metrics to use, what endpoints would be acceptable, how to prove them, enrollment criteria, and the 1000 other minutiae of designing studies and submitting an application to the FDA. (specifics removed by me and XXX’s out)
“In response to the current pandemic caused by a novel coronavirus (SARS-CoV-2) and the associated disease it causes (COVID-19), HHS declared a public health emergency related to COVID-19 on January 31, 2020, and the President declared a national emergency on March 13, 2020. During this time, FDA has been working along with other federal, state, and local agencies and public health officials across the country to help protect public health and address this COVID-19 pandemic.
Due to this current national emergency, FDA has had to allocate resources to COVID-19 related activities, especially for diagnostic tests. CDRH has received more than 6000 pre-Emergency Use Authorization (pre-EUA) and EUA requests and this has led to a significantly increased workload in the Division of Chemistry and Toxicology Devices. To address this unprecedented pandemic IVD workload and enable FDA to address device submissions most needed to combat the pandemic, we have had to pause review of some premarket submissions (e.g., 510(k)s, PMAs, De Novos) even with bringing in additional staff to support COVID-related work. With the limited resources available, we believe it is most important to proceed with review of premarket marketing submissions including the premarket submissions received awaiting review. To accomplish this, however, we are unfortunately unable to review every Q-submission received. At this time, we are unable to review XXXX-related Q-submissions unless they are related to COVID-19, companion diagnostics, a breakthrough device designation request, or have a significant public health impact.
We have received your request for FDA feedback and interaction, and it has been processed as a Q-Submission, XXXXX. However, we regret to inform you that we have determined that your Q-submission does not meet one of these 4 categories mentioned above. Therefore, due to our current resource limitations, we are currently unable to conduct an in-depth review of your submission and provide detailed feedback. We are also unable to hold a teleconference at the present time. With this letter, we are notifying you that your Q-Submission is closed. Unfortunately, we expect that our resource limitation will persist at least through the end of the calendar year which will limit our ability to review XXXXX-related Q-Submissions.
We appreciate that your Q-submission may be related to a future premarket submission. If so, you may submit the relevant marketing submission, and it will be reviewed as we proceed with review of XXXXXX-premarket submissions. We are providing some FDA resources and links that we hope you will be able to use to continue your research and development activities.
as you can see, they are simply not engaging in talk about anything but covid. from what i have seen, “breakthrough status” is being ignored as well and nothing qualifies as “significant public health impact.” not cancer, not debilitating childhood syndrome, not tropical disease, nor drug delivery systems. nada.
there is no disease but “the disease™” covid uber alles.
this means that nearly an entire industry is flying blind.
without this sort of feedback, the odds on an FDA filing or a clinical study not being approvable or acceptable to FDA soar. this back and forth has long been the literal source of the roadmaps. and it’s just gone.
this is leading to clinical trials being canceled.
to spend that kind of money and time when you’re unable to get a sense of whether the regulators are going to accept what you did is just too risky.
this is why drug and device applications are being postponed.
and it’s cascading through a whole industry. the knock on effects are going to last years.
as so many are belatedly learning, you cannot just “turn the world back on” and this is no different. the backlog building up is enormous and it’s all going to bottleneck. these CRL’s can add years to time to market on products and who knows how much additional cost. some of these projects, despite clinical efficacy, will fail as a result.
this is not a make believe game played for goldfish crackers, this is the engine of medical advancement and it has been idled so that we can chase nothing but covid and push massive new products of dubious utility (to the unprecedented profit of a few big companies) while the rest of the industry gets plowed under.
this will, of course, wind up benefiting the big pharma companies a second time as all the stranded assets will be cheap to acquire. (oh, did you think this would be a one act play? not likely.)
medical regulation is, at the best of times, a fraught process subject to all manner of corruption and mis-incentive, but these are not the best of times. this starts to look like full blown regulatory capture and as is every the case in such scenarios, it is small business and we the people who get fed into the corporatist maw.
the FDA seems to have become a wholly owned subsidiary of “covid inc” a public private collaboration of crony capitalism and political preferencing. this is going to pay nasty dividends for a long time to come.
keep one thing in mind:
it was not covid that did this. it was covid policy.
this was a choice.


















Makes you wonder what is happening at the preclinical (animal testing) level if clinical studies are being shut down. I used to work at a preclinical laboratory so I know what all goes into a FDA submission and NORMALLY you have to run several preclinical studies dealing with safety, efficacy, abuse liability (is it addictive), neurotoxicity, reproductive and fertility, carcinogenicity, cardiovascular and pulmonary, just to name a few, before it is ever tested on humans. I am not aware of any preclinical studies that have been done on these vaccines. When I left the industry in 2017 there was no talk of mRNA vaccines being developed. If there had been, my laboratory would have been on the frontlines of this development. Seeing as it takes between 6-10 years to successfully bring a new drug to market, I am deeply deeply suspicious that shortcuts have been taken with these vaccines, and this is what I tell people when they ask why I am not getting any of them. I spent 37 years in this business and I was in a position to know.
COVID epidemiology data clearly do not support a pandemic, as I showed in my book The Defeat Of COVID, but political events since early 2020 do show a clear, incremental path toward medical tyranny.
However, that doesn't mean that Scott Gottlieb was a saint for having left the FDA earlier. The FDA has long had an 80% personnel revolving door with pharma & fox-in-henhouse role for many decades. Gottlieb, predictably, moved from FDA to Pfizer's board, which may be somewhere down around the 9th ring of hell, but I'm dusty on my Dante there.
Your industry, gato malo, may not have suffered under Gottlieb, but such shenanigans as definitional changes that pushed nutrients into a "drug" category (503A & 503B) stomped on naturopathic doctors and compounding pharmacies, and happened under Gottlieb's watch, which served to make unfair regulatory competition, and was a clear violation of antitrust laws. Gottlieb got away with it, because FDA benefactor corporations have $$$$$ lawyers, and all we in natural medicine have is the gratitude of our patients and few $.